Itraconazole
 | |
| Clinical data | |
|---|---|
| Trade names | Sporanox, Sporaz, Orungal, others |
| Other names | ITZ |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692049 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, solution), vaginal suppository, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~55%, maximal if taken with full meal |
| Protein binding | 99.8% |
| Metabolism | Extensive in liver (CYP3A4) |
| Metabolites | Hydroxy-itraconazole, keto-itraconazole, N-desalkyl-itraconazole[4] |
| Elimination half-life | 21 hours |
| Excretion | Kidney (35%), faeces (54%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.596 |
| Chemical and physical data | |
| Formula | C35H38Cl2N8O4 |
| Molar mass | 705.64 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 165[6] °C (329 °F) |
| Solubility in water | 7.8 ± 0.4 × 10−6 mol/L (pH 1.6)[6] mg/mL (20 °C) |
| |
| |
| (verify) | |
Itraconazole, sometimes abbreviated ITZ, is an antifungal medication used to treat a number of fungal infections.[7] This includes aspergillosis, blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis.[7] It may be given by mouth or intravenously.[7]
Common side effects include nausea, diarrhea, abdominal pain, rash, and headache.[7] Severe side effects may include liver problems, heart failure, Stevens–Johnson syndrome and allergic reactions including anaphylaxis.[7] It is unclear if use during pregnancy or breastfeeding is safe.[1] It is in the triazole family of medications.[7] It stops fungal growth by affecting the cell membrane or affecting their metabolism.[7]
Itraconazole was patented in 1978 and approved for medical use in the United States in 1992.[7][8] It is on the World Health Organization's List of Essential Medicines.[9]
Recent research works suggest itraconazole (ITZ) could also be used in the treatment of cancer by inhibiting the hedgehog pathway[10] in a similar way to sonidegib.
Medical uses
[edit]Itraconazole has a broader spectrum of activity than fluconazole (but not as broad as voriconazole or posaconazole). In particular, it is active against Aspergillus, which fluconazole is not. It is also licensed for use in blastomycosis, sporotrichosis, histoplasmosis, and onychomycosis. Itraconazole is over 99% protein-bound and has virtually no penetration into cerebrospinal fluid. Therefore, it should not be used to treat meningitis or other central nervous system infections.[11] According to the Johns Hopkins Abx Guide, it has "negligible CSF penetration, however treatment has been successful for cryptococcal and coccidioidal meningitis".[12]
It is also prescribed for systemic infections, such as aspergillosis, candidiasis, and cryptococcosis, where other antifungal drugs are inappropriate or ineffective.[citation needed]
Itraconazole has been explored as an anticancer agent for patients with basal cell carcinoma, non-small cell lung cancer, glioblastoma and prostate cancer, [13][14] For example, in a phase II study involving men with advanced prostate cancer, high-dose itraconazole (600 mg/day) was associated with significant PSA responses and a delay in tumor progression. Itraconazole also showed activity in a phase II trial in men with non-small cell lung cancer when it was combined with the chemotherapy agent, pemetrexed.[15][16][17] A recent review also highlights its use topically and orally in conjunction with other chemotherapeutic agents for advanced and metastatic basal cell carcinomas that cannot be treated surgically.[18]
Available forms
[edit]Itraconazole is produced as blue 22 mm (0.87 in) capsules with tiny 1.5 mm (0.059 in) blue pellets inside. Each capsule contains 100 mg and is usually taken twice a day at twelve-hour intervals. The Sporanox brand of itraconazole has been developed and marketed by Janssen Pharmaceutica, a subsidiary of Johnson & Johnson.[citation needed] The three-layer structure of these blue capsules is complex because itraconazole is insoluble and is sensitive to pH. The complicated procedure not only requires a specialized machine to create it, but also the method used has manufacturing problems. Also, the pill is quite large, making it difficult for many patients to swallow. Parts of the processes of creating Sporanox were discovered by the Korean Patent Laid-open No. 10-2001-2590.[19] The tiny blue pellets contained in the capsule are manufactured in Beerse, Belgium.[19][20]
The oral solution is better absorbed. The cyclodextrin contained in the oral solution can cause an osmotic diarrhea, and if this is a problem, then half the dose can be given as oral solution and half as capsule to reduce the amount of cyclodextrin given.[citation needed] "Sporanox" itraconazole capsules should always be taken with food, as this improves absorption, however the manufacturers of "Lozanoc" assert that it may be taken "without regard to meals".[21] Itraconazole oral solution should be taken an hour before food, or two hours after food (and likewise if a combination of capsules and oral solution are used). Itraconazole may be taken with orange juice or cola, as absorption is also improved by acid. Absorption of itraconazole is impaired when taken with an antacid, H2 blocker or proton pump inhibitor.[citation needed]
Side effects
[edit]Itraconazole is a relatively well-tolerated drug (although not as well tolerated as fluconazole or voriconazole) and the range of adverse effects it produces is similar to the other azole antifungals:[22]
- elevated alanine aminotransferase levels are found in 4% of people taking itraconazole
- "small but real risk" of developing congestive heart failure[22]
- liver failure, sometimes fatal
The cyclodextrin used to make the syrup preparation can cause diarrhea. Side effects that may indicate a greater problem include:[citation needed]
Interactions
[edit]The following drugs should not be taken with itraconazole:[23]
- amiodarone (Cordarone);[24]
- cisapride
- dofetilide
- nisoldipine
- alcohol
- pimozide
- quinidine
- lurasidone
- lovastatin or simvastatin
- midazolam or triazolam
- ergot medicines such as
Pharmacology
[edit]Pharmacodynamics
[edit]The mechanism of action of itraconazole is the same as the other azole antifungals: it inhibits the fungal-mediated synthesis of ergosterol, via inhibition of lanosterol 14α-demethylase. Because of its ability to inhibit cytochrome P450 3A4 CC-3, caution should be used when considering interactions with other medications.[25]
Itraconazole is pharmacologically distinct from other azole antifungal agents in that it is the only inhibitor in this class that has been shown to inhibit both the hedgehog signaling pathway[26][27] and angiogenesis.[28][29] These distinct activities are unrelated to inhibition of the cytochrome P450 lanosterol 14 alpha-demethylase and the exact molecular targets responsible remain unidentified. Functionally, the antiangiogenic activity of itraconazole has been shown to be linked to inhibition of glycosylation, VEGFR2 phosphorylation,[29] trafficking,[30] and cholesterol biosynthesis pathways.[28] Evidence suggests the structural determinants for inhibition of hedgehog signaling by itraconazole are recognizably different from those associated with antiangiogenic activity.[31]
Pharmacokinetics
[edit]Itraconazole, like cyclosporine, quinidine, and clarithromycin, can inhibit P-glycoprotein, causing drug interactions by reducing elimination and increasing absorption of organic cation drugs. With conventional itraconazole preparations serum levels can vary greatly between patients, often resulting in serum concentrations lower than the therapeutic index.[32] It has therefore been conventionally advised that patients take itraconazole after a fatty meal rather than prior to eating.[33][34]
A product (Lozanoc) licensed through the European union decentralised procedure[35] has increased bioavailability, decreased sensitivity to co ingestion of food, and hence decreased variability of serum levels.
Chemistry
[edit]
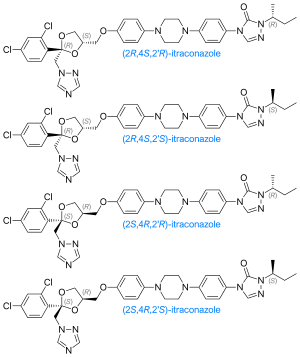
The itraconazole molecule has three chiral carbons. The two chiral centers in the dioxolane ring are fixed in relation to one another, and the triazolomethylene and aryloxymethylene dioxolane-ring substituents are always cis to each other. The clinical formulation is a 1:1:1:1 mixture of four stereoisomers (two enantiomeric pairs).[36][37]
History
[edit]Itraconazole was approved for medical use in the United States in 1992.[38]
It was designated an orphan drug by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).[39][40][41][42][43]
References
[edit]- ^ a b "Itraconazole Use During Pregnancy". Drugs.com. 20 March 2019. Retrieved 15 May 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Sporanox 10 mg/mL Oral Solution - Summary of Product Characteristics (SmPC)". (emc). 1 February 2018. Retrieved 15 May 2020.
- ^ Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE (October 2004). "Role of itraconazole metabolites in CYP3A4 inhibition". Drug Metabolism and Disposition. 32 (10): 1121–1131. doi:10.1124/dmd.104.000315. PMID 15242978. S2CID 6941636.
- ^ "Sporanox (itraconazole) Capsules. Full Prescribing Information" (PDF). Janssen Pharmaceuticals, Inc. Archived from the original (PDF) on 17 May 2018. Retrieved 28 August 2016.
- ^ a b Vasilev NA, Surov AO, Voronin AP, Drozd KV, Perlovich GL (April 2021). "Novel cocrystals of itraconazole: Insights from phase diagrams, formation thermodynamics and solubility". International Journal of Pharmaceutics. 599: 120441. doi:10.1016/j.ijpharm.2021.120441. PMID 33675927. S2CID 232135660.
- ^ a b c d e f g h "Itraconazole". The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 503. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Li K, Fang D, Xiong Z, Luo R (2019). "Inhibition of the hedgehog pathway for the treatment of cancer using Itraconazole". OncoTargets and Therapy. 12: 6875–6886. doi:10.2147/OTT.S223119. PMC 6711563. PMID 31692536.
- ^ Gilbert DN, Moellering, RC, Eliopoulos GM, Sande MA (2006). The Sanford Guide to antimicrobial therapy. Antimicrobial Therapy, Incorporated. ISBN 978-1-930808-30-0.[page needed]
- ^ Pham P, Bartlett JG (24 July 2007). "Itraconazole". Johns Hopkins. Archived from the original on 28 November 2007.
- ^ Halatsch ME, Kast RE, Karpel-Massler G, Mayer B, Zolk O, Schmitz B, Scheuerle A, Maier L, Bullinger L, Mayer-Steinacker R, Schmidt C, Zeiler K, Elshaer Z, Panther P, Schmelzle B, Hallmen A, Dwucet A, Siegelin MD, Westhoff MA, Beckers K, Bouche G, Heiland T (2021). "A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3". Neurooncol Adv. 3 (1): vdab075. doi:10.1093/noajnl/vdab075. PMC 8349180. PMID 34377985.
- ^ "Search results for Itraconazole". ClinicalTrials.gov. U.S. National Institutes of Health.
- ^ Aftab BT, Dobromilskaya I, Liu JO, Rudin CM (2011). "Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer". Cancer Research. 71 (21): 6764–72. doi:10.1158/0008-5472.CAN-11-0691. PMC 3206167. PMID 21896639.
- ^ Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS, Zhao M, Mendonca J, Kachhap SK, Rudek MA, Carducci MA (2013). "Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer". The Oncologist. 18 (2): 163–173. doi:10.1634/theoncologist.2012-314. PMC 3579600. PMID 23340005.
- ^ Rudin CM, Brahmer JR, Juergens RA, Hann CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P, Liu JO (May 2013). "Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer". Journal of Thoracic Oncology. 8 (5): 619–623. doi:10.1097/JTO.0b013e31828c3950. PMC 3636564. PMID 23546045.
- ^ Ip KH, McKerrow K (2021). "Itraconazole in the treatment of basal cell carcinoma: A case-based review of the literature". Australasian Journal of Dermatology. 62 (3): 394–7. doi:10.1111/ajd.13655. PMID 34160824. S2CID 235608763.
- ^ a b US 20050226924, Lee KH, Park ES, Chi SC, "Composition comprising Itraconazole for oral administration", published 13 October 2005, assigned to FDL Inc.
- ^ "Sporanox (Itraconazole Capsules)" (PDF). Janssen. June 2006. Archived from the original (PDF) on 5 July 2008.
- ^ "SUBA Bioavailability Technology". Mayne Pharma Group.
- ^ a b "The Safety of Sporanox Capsules and Lamisil Tablets for the Treatment of Onychomycosis". FDA Public Health Advisory. 9 May 2001. Archived from the original on 28 May 2009. Retrieved 10 August 2006.
- ^ "Sporanox (Itraconazole) Capsules". Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research. U.S. Food and Drug Administration (FDA). Archived from the original on 26 October 2016. Retrieved 16 December 2019.
- ^ Tsimogianni AM, Andrianakis I, Betrosian A, Douzinas E (July 2011). "Cardiac arrest provoked by itraconazole and amiodarone interaction: a case report". Journal of Medical Case Reports. 5: 333. doi:10.1186/1752-1947-5-333. PMC 3161953. PMID 21801420.
- ^ Trevor AJ, Katzung BG, Kruidering-Hall M (2015). Katzung & Trevor's Pharmacology: Examination & Board Review (Eleventh ed.). New York: McGraw Hill Medical. p. 397. ISBN 978-0-07-182635-8.
- ^ Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA (2010). "Itraconazole, a Commonly Used Antifungal that Inhibits Hedgehog Pathway Activity and Cancer Growth". Cancer Cell. 17 (4): 388–99. doi:10.1016/j.ccr.2010.02.027. PMC 4039177. PMID 20385363.
- ^ Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Beachy PA, Rudin CM (2013). "Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists". Cancer Cell. 23 (1): 23–34. doi:10.1016/j.ccr.2012.11.017. PMC 3548977. PMID 23291299.
- ^ a b Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Liu JO (2007). "Inhibition of Angiogenesis by the Antifungal Drug Itraconazole". ACS Chemical Biology. 2 (4): 263–70. doi:10.1021/cb600362d. PMID 17432820.
- ^ a b Aftab BT, Dobromilskaya I, Liu JO, Rudin CM (2011). "Itraconazole Inhibits Angiogenesis and Tumor Growth in Non-Small Cell Lung Cancer". Cancer Research. 71 (21): 6764–72. doi:10.1158/0008-5472.CAN-11-0691. PMC 3206167. PMID 21896639.
- ^ Xu J, Dang Y, Ren YR, Liu JO (2010). "Cholesterol trafficking is required for mTOR activation in endothelial cells". Proceedings of the National Academy of Sciences. 107 (10): 4764–9. Bibcode:2010PNAS..107.4764X. doi:10.1073/pnas.0910872107. PMC 2842052. PMID 20176935.
- ^ Shi W, Nacev BA, Aftab BT, Head S, Rudin CM, Liu JO (2011). "Itraconazole Side Chain Analogues: Structure–Activity Relationship Studies for Inhibition of Endothelial Cell Proliferation, Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Glycosylation, and Hedgehog Signaling". Journal of Medicinal Chemistry. 54 (20): 7363–74. doi:10.1021/jm200944b. PMC 3307530. PMID 21936514.
- ^ Patterson TF, Peters J, Levine SM, Anzueto A, Bryan CL, Sako EY, Miller OL, Calhoon JH, Rinaldi MG (1996). "Systemic availability of itraconazole in lung transplantation". Antimicrob. Agents Chemother. 40 (9): 2217–20. doi:10.1128/AAC.40.9.2217. PMC 163504. PMID 8878612.
- ^ Fraga Fuentes MD, García Díaz B, de Juana Velasco P, Bermejo Vicedo MT (1997). "[Influence of foods on the absorption of antimicrobial agents]". Nutr Hosp (in Spanish). 12 (6): 277–88. PMID 9477653.
- ^ Barone JA, Koh JG, Bierman RH, Colaizzi JL, Swanson KA, Gaffar MC, Moskovitz BL, Mechlinski W, Van de Velde V (1993). "Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers". Antimicrob. Agents Chemother. 37 (4): 778–84. doi:10.1128/aac.37.4.778. PMC 187759. PMID 8388198.
- ^ "Lozanoc 50 Mg Hard Capsules (Itraconazole)" (PDF). Public Assessment Report Decentralised Procedure. UK Medicines and Health Care Products Regulatory Agency.
- ^ Kunze KL, Nelson WL, Kharasch ED, Thummel KE, Isoherranen N (April 2006). "Stereochemical aspects of itraconazole metabolism in vitro and in vivo". Drug Metabolism and Disposition. 34 (4): 583–590. doi:10.1124/dmd.105.008508. PMID 16415110. S2CID 189994.
- ^ "Itraconazole on Drugs.com". Drugs.com. Retrieved 28 August 2016.
- ^ "Itraconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 19 May 2016. Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 16 August 2016. Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 30 October 2008. Retrieved 15 May 2020.
- ^ "EU/3/17/1901". European Medicines Agency (EMA). 23 August 2017. Retrieved 15 May 2020.
- ^ "EU/3/18/2024". European Medicines Agency (EMA). 25 May 2018. Retrieved 15 May 2020.
External links
[edit] Media related to Itraconazole at Wikimedia Commons
Media related to Itraconazole at Wikimedia Commons
- 1,2,4-Triazol-3-ones
- Belgian inventions
- Chloroarenes
- CYP3A4 inhibitors
- Dioxolanes
- Drugs developed by Johnson & Johnson
- Janssen Pharmaceutica
- Lanosterol 14α-demethylase inhibitors
- Orphan drugs
- Piperazines
- Triazole antifungals
- World Health Organization essential medicines
- Phenylethanolamine ethers
- Sec-Butyl compounds
